Exo and endo isomers.
If you’re searching for exo and endo isomers images information connected with to the exo and endo isomers keyword, you have come to the right site. Our site frequently provides you with hints for seeing the highest quality video and picture content, please kindly search and locate more enlightening video articles and graphics that match your interests.
 Chapter 16 17 Discussion Endo And Exo Are Meaningless Without Substitutents To Provide Frame Of Reference Meso Identical Ppt Download From slideplayer.com
Chapter 16 17 Discussion Endo And Exo Are Meaningless Without Substitutents To Provide Frame Of Reference Meso Identical Ppt Download From slideplayer.com
In the anaesthetized rat the exo- and endo-isomers of 2-aminobenzonorbornene and their N-methyl derivatives all had similar pressor activities though the successive injections of the two exo-derivatives suggested an additional alpha-adrenoceptor blocking activity. We report here an effective large-scale separation of exo- and endo-isomers using a partial recycling technique with HPLC. Other strong dienophiles also add to furan 88. The steric demand of both 7-carba and 7-oxanorbornene derivatives is very similar and it is not immediately apparent to us what causes the observed difference in initiation kinetics.
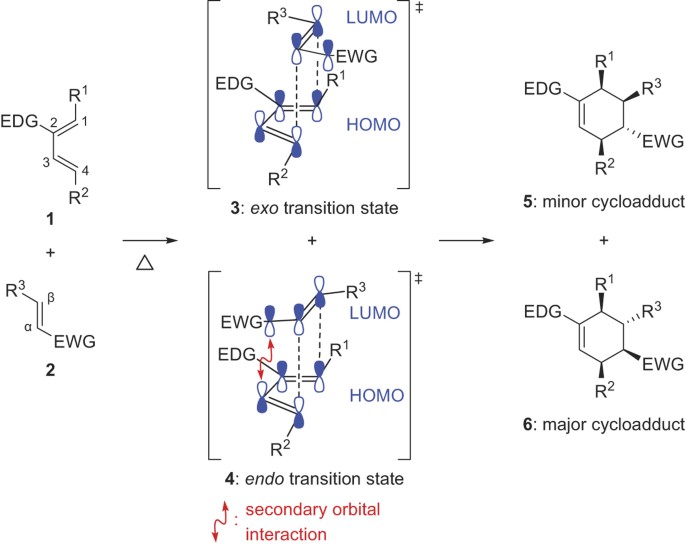
As a general rule the endo isomer is more stable when the substituents are H CH 3 and electrodonating groups while the exo isomer is strongly preferred when the substituents are good electron withdrawing groups like CN compounds 5 6df and COOCH 3 compounds 7 8.
The increases in mean arterial blood pressure caused by noradrenaline NA amphetamine Am the exo- and endo-isomers of 2-aminobenzonorbornene 1 exo 1 endo and the exo- and endo-isomers of Nmethyl-2-aminobenzonorbornene 2 exo 2 endo. Endo and exo isomers Furan and maleic anhydride undergo the Diels-Alder reaction to form the tricycHc 1 1 adduct 7-oxabicyclo 221hept-5-ene-23-dicarboxyHc anhydride 4 in exceUent yield. The exoendo isomerization of 25-dimethoxybenzaldehyde was theoretically studied by density functional theory DFT to examine its favored conformers via sp2sp2 single rotation. This is tentatively assigned as the endo isomer and on base treatment it isomerizes to a new compound identified as the exo isomer. Giga-fren In both cases rearrangement to the corresponding 88-dimethyl-tricyclo421024nonan-7-one 11 18 was found with the exo isomer reacting approximately 8.
 Source: youtube.com
Source: youtube.com
Here longest and shortest refer to the number of atoms that. Endoexo isomerism is a special type of stereoisomerism found in organic compounds with a substituent on a bridged ring system. The prefix endo is reserved for the isomer with the substituent located closest or syn to the longest bridge. The exoendo isomerization of 25-dimethoxybenzaldehyde was theoretically studied by density functional theory DFT to examine its favored conformers via sp2sp2 single rotation. The prefix exo is reserved for the isomer with the substituent located farthest or anti to the longest bridge.
As a general rule the endo isomer is more stable when the substituents are H CH 3 and electrodonating groups while the exo isomer is strongly preferred when the substituents are good electron withdrawing groups like CN compounds 5 6df and COOCH 3 compounds 7 8.
A requirement for distinct endo and exo-stereoisomers is two different groups a reacting site in both diene and dienophile. Giga-fren In both cases rearrangement to the corresponding 88-dimethyl-tricyclo421024nonan-7-one 11 18 was found with the exo isomer reacting approximately 8. We report here an effective large-scale separation of exo- and endo-isomers using a partial recycling technique with HPLC. The actual product formed is the endo adduct.
 Source: nature.com
Source: nature.com
Heating of the 21 adduct 13 gives a mixture containing the endo-anti-exo isomer 15 the exo-anti-exo isomer 16 and 13. This is because although the hydrogens of the maleic anhydride must be cis in the product there are two possible arrangements where this is true. Exoendo and exoexo isomers of 69- PMe2Ph2-arachno-B10H12 and its halogenated derivatives. Molecular structures of exoendo- and exoexo-69- PMe2Ph2-arachno-B10H12 and exo-6endo-9- PMe2Ph2-2-Br-arachno-B10H11 - Journal of the Chemical Society Dalton Transactions RSC Publishing Issue 24 1997.
 Source: slideplayer.com
Source: slideplayer.com
As a general rule the endo isomer is more stable when the substituents are H CH 3 and electrodonating groups while the exo isomer is strongly preferred when the substituents are good electron withdrawing groups like CN compounds 5 6df and COOCH 3 compounds 7 8. Both isomers were docked against 1BNA DNA to elucidate their binding ability and the DFT-computed structural parameters results were matched with the X-ray diffraction XRD crystallographic. The exoendo isomerization of 25-dimethoxybenzaldehyde was theoretically studied by density functional theory DFT to examine its favored conformers via sp2sp2 single rotation. A requirement for distinct endo and exo-stereoisomers is two different groups a reacting site in both diene and dienophile.
 Source: j-tradition.com
Source: j-tradition.com
In the anaesthetized rat the exo- and endo-isomers of 2-aminobenzonorbornene and their N-methyl derivatives all had similar pressor activities though the successive injections of the two exo-derivatives suggested an additional alpha-adrenoceptor blocking activity. Initially G3 reacted mainly with the exo isomer 30 endo-carbene 70 exo-carbene and over time 60 min converted exclusively to the endo carbene after complete consumption of the exo-derivative. Certain structural requirements must be met in the diene and dienophile for the generation of endo and exo diastereomers. The increases in mean arterial blood pressure caused by noradrenaline NA amphetamine Am the exo- and endo-isomers of 2-aminobenzonorbornene 1 exo 1 endo and the exo- and endo-isomers of Nmethyl-2-aminobenzonorbornene 2 exo 2 endo.
Endo-exo isomerism is a special type of isomerism found in organic compounds with a substituent on a bridged ring system. This is tentatively assigned as the endo isomer and on base treatment it isomerizes to a new compound identified as the exo isomer. The actual product formed is the endo adduct. This is because although the hydrogens of the maleic anhydride must be cis in the product there are two possible arrangements where this is true.
The prefix endo is reserved for the isomer with the substituent located closest or syn to the longest bridge.
Heating of the 21 adduct 13 gives a mixture containing the endo-anti-exo isomer 15 the exo-anti-exo isomer 16 and 13. The prefix exo is reserved for the isomer with the substituent located farthest or anti to the longest bridge. This is because although the hydrogens of the maleic anhydride must be cis in the product there are two possible arrangements where this is true. This is tentatively assigned as the endo isomer and on base treatment it isomerizes to a new compound identified as the exo isomer. Endoexo isomerism is a special type of stereoisomerism found in organic compounds with a substituent on a bridged ring system.
 Source: nature.com
Source: nature.com
Since each chiral centre could have two possible configurations there are sixteen possible stereoisomers that could result in the reaction shown above. Exoendo and exoexo isomers of 69- PMe2Ph2-arachno-B10H12 and its halogenated derivatives. The actual product formed is the endo adduct. Endo and exo isomers Furan and maleic anhydride undergo the Diels-Alder reaction to form the tricycHc 1 1 adduct 7-oxabicyclo 221hept-5-ene-23-dicarboxyHc anhydride 4 in exceUent yield. In the multi-stage synthesis of chiral norbornylcyclohexanones in our laboratories it became necessary to separate at an intermediate stage the exo- and endoisomers of d- and 1-2-methyl-4-556e-vo-trimethyl-2-norbornyllanisole I and 11 which had been prepared as.
The 13 C NMR spectra of the exo exo- and exo endo-isomers of tetracyclo 6210 271 36 dodecane were studied. We report here an effective large-scale separation of exo- and endo-isomers using a partial recycling technique with HPLC. Heating of the 21 adduct 13 gives a mixture containing the endo-anti-exo isomer 15 the exo-anti-exo isomer 16 and 13. Giga-fren In both cases rearrangement to the corresponding 88-dimethyl-tricyclo421024nonan-7-one 11 18 was found with the exo isomer reacting approximately 8.
The endo and exo products are really two different diastereomers.
The chemical shift of the bridge carbon depends on the configuration of the geometric isomer and can be used as a criterion to identify the stereoisomers in polycyclic systems with a norbornane fragment. Endo-exo isomerism is a special type of isomerism found in organic compounds with a substituent on a bridged ring system. The Diels-Alder reaction between cyclopentadiene and maleic anhydride can produce two possible products the endo and the exo adducts. Here longest and shortest refer to the number of atoms that.
 Source: j-tradition.com
Source: j-tradition.com
Endo and exo isomers Furan and maleic anhydride undergo the Diels-Alder reaction to form the tricycHc 1 1 adduct 7-oxabicyclo 221hept-5-ene-23-dicarboxyHc anhydride 4 in exceUent yield. In the anaesthetized rat the exo- and endo-isomers of 2-aminobenzonorbornene and their N-methyl derivatives all had similar pressor activities though the successive injections of the two exo-derivatives suggested an additional alpha-adrenoceptor blocking activity. Other strong dienophiles also add to furan 88. The prefix exo is reserved for the isomer with the substituent located farthest or anti to the longest bridge.
 Source: slideplayer.com
Source: slideplayer.com
The Diels-Alder reaction between cyclopentadiene and maleic anhydride can produce two possible products the endo and the exo adducts. Heating of the 21 adduct 13 gives a mixture containing the endo-anti-exo isomer 15 the exo-anti-exo isomer 16 and 13. Initially G3 reacted mainly with the exo isomer 30 endo-carbene 70 exo-carbene and over time 60 min converted exclusively to the endo carbene after complete consumption of the exo-derivative. Endo and exo isomers Furan and maleic anhydride undergo the Diels-Alder reaction to form the tricycHc 1 1 adduct 7-oxabicyclo 221hept-5-ene-23-dicarboxyHc anhydride 4 in exceUent yield.
 Source: nature.com
Source: nature.com
Giga-fren In both cases rearrangement to the corresponding 88-dimethyl-tricyclo421024nonan-7-one 11 18 was found with the exo isomer reacting approximately 8. The chemical shift of the bridge carbon depends on the configuration of the geometric isomer and can be used as a criterion to identify the stereoisomers in polycyclic systems with a norbornane fragment. Giga-fren In both cases rearrangement to the corresponding 88-dimethyl-tricyclo421024nonan-7-one 11 18 was found with the exo isomer reacting approximately 8. The prefix endo is reserved for the isomer with the substituent located closest or syn to the longest bridge.
Both isomers were docked against 1BNA DNA to elucidate their binding ability and the DFT-computed structural parameters results were matched with the X-ray diffraction XRD crystallographic.
Endoexo isomerism is a special type of stereoisomerism found in organic compounds with a substituent on a bridged ring system. The prefix exo is reserved for the isomer with the substituent located farthest or anti to the longest bridge. The actual product formed is the endo adduct. The actions of these rigid. If you think about it you can see that when two rings fuse together to make a third four new stereocenters can be created.
 Source: exo2020reborn.blogspot.com
Source: exo2020reborn.blogspot.com
The increases in mean arterial blood pressure caused by noradrenaline NA amphetamine Am the exo- and endo-isomers of 2-aminobenzonorbornene 1 exo 1 endo and the exo- and endo-isomers of Nmethyl-2-aminobenzonorbornene 2 exo 2 endo. Certain structural requirements must be met in the diene and dienophile for the generation of endo and exo diastereomers. This is tentatively assigned as the endo isomer and on base treatment it isomerizes to a new compound identified as the exo isomer. If you think about it you can see that when two rings fuse together to make a third four new stereocenters can be created. Endo-exo isomerism is a special type of isomerism found in organic compounds with a substituent on a bridged ring system.
Endo-exo isomerism is a special type of isomerism found in organic compounds with a substituent on a bridged ring system.
The prefix exo is reserved for the isomer with the substituent located farthest or anti to the longest bridge. A requirement for distinct endo and exo-stereoisomers is two different groups a reacting site in both diene and dienophile. The actual product formed is the endo adduct. Here longest and shortest refer to the number of atoms that.
 Source: onlinelibrary.wiley.com
Source: onlinelibrary.wiley.com
Heating of the 21 adduct 13 gives a mixture containing the endo-anti-exo isomer 15 the exo-anti-exo isomer 16 and 13. The increases in mean arterial blood pressure caused by noradrenaline NA amphetamine Am the exo- and endo-isomers of 2-aminobenzonorbornene 1 exo 1 endo and the exo- and endo-isomers of Nmethyl-2-aminobenzonorbornene 2 exo 2 endo. Endo-exo isomerism is a special type of isomerism found in organic compounds with a substituent on a bridged ring system. Initially G3 reacted mainly with the exo isomer 30 endo-carbene 70 exo-carbene and over time 60 min converted exclusively to the endo carbene after complete consumption of the exo-derivative.
 Source: j-tradition.com
Source: j-tradition.com
We report here an effective large-scale separation of exo- and endo-isomers using a partial recycling technique with HPLC. We report here an effective large-scale separation of exo- and endo-isomers using a partial recycling technique with HPLC. A requirement for distinct endo and exo-stereoisomers is two different groups a reacting site in both diene and dienophile. The exoendo isomerization of 25-dimethoxybenzaldehyde was theoretically studied by density functional theory DFT to examine its favored conformers via sp2sp2 single rotation.
 Source: j-tradition.com
Source: j-tradition.com
The prefix endo is reserved for the isomer with the substituent located closest or syn to the longest bridge. The prefix endo is reserved for the isomer with the substituent located closest or syn to the longest bridge. Both isomers were docked against 1BNA DNA to elucidate their binding ability and the DFT-computed structural parameters results were matched with the X-ray diffraction XRD crystallographic. In the multi-stage synthesis of chiral norbornylcyclohexanones in our laboratories it became necessary to separate at an intermediate stage the exo- and endoisomers of d- and 1-2-methyl-4-556e-vo-trimethyl-2-norbornyllanisole I and 11 which had been prepared as.
The actions of these rigid.
Initially G3 reacted mainly with the exo isomer 30 endo-carbene 70 exo-carbene and over time 60 min converted exclusively to the endo carbene after complete consumption of the exo-derivative. The exoendo isomerization of 25-dimethoxybenzaldehyde was theoretically studied by density functional theory DFT to examine its favored conformers via sp2sp2 single rotation. Initially G3 reacted mainly with the exo isomer 30 endo-carbene 70 exo-carbene and over time 60 min converted exclusively to the endo carbene after complete consumption of the exo-derivative. The steric demand of both 7-carba and 7-oxanorbornene derivatives is very similar and it is not immediately apparent to us what causes the observed difference in initiation kinetics. Certain structural requirements must be met in the diene and dienophile for the generation of endo and exo diastereomers.
 Source: exo2020reborn.blogspot.com
Source: exo2020reborn.blogspot.com
The prefix endo is reserved for the isomer with the substituent located closest or syn to the longest bridge. The chemical shift of the bridge carbon depends on the configuration of the geometric isomer and can be used as a criterion to identify the stereoisomers in polycyclic systems with a norbornane fragment. Other strong dienophiles also add to furan 88. The prefix endo is reserved for the isomer with the substituent located closest or syn to the longest bridge. The 13 C NMR spectra of the exo exo- and exo endo-isomers of tetracyclo 6210 271 36 dodecane were studied.
This is because although the hydrogens of the maleic anhydride must be cis in the product there are two possible arrangements where this is true.
This is because although the hydrogens of the maleic anhydride must be cis in the product there are two possible arrangements where this is true. The Diels-Alder reaction between cyclopentadiene and maleic anhydride can produce two possible products the endo and the exo adducts. The 13 C NMR spectra of the exo exo- and exo endo-isomers of tetracyclo 6210 271 36 dodecane were studied. Endo and exo isomers Furan and maleic anhydride undergo the Diels-Alder reaction to form the tricycHc 1 1 adduct 7-oxabicyclo 221hept-5-ene-23-dicarboxyHc anhydride 4 in exceUent yield.
 Source: j-tradition.com
Source: j-tradition.com
Molecular structures of exoendo- and exoexo-69- PMe2Ph2-arachno-B10H12 and exo-6endo-9- PMe2Ph2-2-Br-arachno-B10H11 - Journal of the Chemical Society Dalton Transactions RSC Publishing Issue 24 1997. Here longest and shortest refer to the number of atoms that. The prefix exo is reserved for the isomer with the substituent located farthest or anti to the longest bridge. The chemical shift of the bridge carbon depends on the configuration of the geometric isomer and can be used as a criterion to identify the stereoisomers in polycyclic systems with a norbornane fragment. This is because although the hydrogens of the maleic anhydride must be cis in the product there are two possible arrangements where this is true.
 Source: j-tradition.com
Source: j-tradition.com
Endo and exo isomers Furan and maleic anhydride undergo the Diels-Alder reaction to form the tricycHc 1 1 adduct 7-oxabicyclo 221hept-5-ene-23-dicarboxyHc anhydride 4 in exceUent yield. The actions of these rigid. The chemical shift of the bridge carbon depends on the configuration of the geometric isomer and can be used as a criterion to identify the stereoisomers in polycyclic systems with a norbornane fragment. The prefix endo is reserved for the isomer with the substituent located closest or syn to the longest bridge. Molecular structures of exoendo- and exoexo-69- PMe2Ph2-arachno-B10H12 and exo-6endo-9- PMe2Ph2-2-Br-arachno-B10H11 - Journal of the Chemical Society Dalton Transactions RSC Publishing Issue 24 1997.
 Source: j-tradition.com
Source: j-tradition.com
Endo-exo isomerism is a special type of isomerism found in organic compounds with a substituent on a bridged ring system. Endoexo isomerism is a special type of stereoisomerism found in organic compounds with a substituent on a bridged ring system. The increases in mean arterial blood pressure caused by noradrenaline NA amphetamine Am the exo- and endo-isomers of 2-aminobenzonorbornene 1 exo 1 endo and the exo- and endo-isomers of Nmethyl-2-aminobenzonorbornene 2 exo 2 endo. If you think about it you can see that when two rings fuse together to make a third four new stereocenters can be created. The prefix endo is reserved for the isomer with the substituent located closest or syn to the longest bridge.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title exo and endo isomers by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





