Endo and exo isomers examples.
If you’re searching for endo and exo isomers examples images information related to the endo and exo isomers examples topic, you have pay a visit to the ideal blog. Our site always provides you with suggestions for viewing the maximum quality video and image content, please kindly search and locate more informative video articles and images that match your interests.
 Diels Alder Reaction Of Pericyclic Reactions Dienes And Dienophiles Ryosuke University From j-tradition.com
Diels Alder Reaction Of Pericyclic Reactions Dienes And Dienophiles Ryosuke University From j-tradition.com
A detergent comprising tetrahydrodicyclopentadiene in which the endoexo isomer ratio by weight is from 7030 to 0100. It is possible to convert the endo isomer 5b to the exo isomer 5a by an epimerization process through the enol. Giga-fren In sharp contrast to their 321024 analogs the endo isomer is stable while the exo readily rearranges to. The ENDO product is the one where the outside groups on the diene are on the SAME side of the 6-membered ring as the electron withdrawing group EWG.
This diastereoisomer is the less stable one but is formed.
There is usually a temperature change. C-7 in example below it is given the description exo. Here longest and shortest refer to the. Giga-fren In sharp contrast to their 321024 analogs the endo isomer is stable while the exo readily rearranges to. The endo- to exo-isomerization of dicyclopentadiene was performed in liquid phase using acidic zeolites.
 Source: j-tradition.com
Source: j-tradition.com
A detergent comprising tetrahydrodicyclopentadiene in which the endoexo isomer ratio by weight is from 7030 to 0100. Translations in context of endo in French-English from Reverso Context. Two examples follow which are drawn to emphasize how suprafacial addition occurs. If you think about it you can see that when two rings fuse together to make a third four new stereocenters can be created. Beta and Y-type zeolites exhibit higher activity because of their large three-dimensional channels.
Giga-fren In sharp contrast to their 321024 analogs the endo isomer is stable while the exo readily rearranges to.
Surface passivation of Hβ confirms that the reaction proceeds in the inner channels. The prefix endo is reserved for the isomer with the substituent located closest or syn to the longest bridge. Among the zeolites tested the activity order is Hβ HY HUSY HZSM-5 H-mordenite. It is given the description endo.
 Source: j-tradition.com
Source: j-tradition.com
Among the zeolites tested the activity order is Hβ HY HUSY HZSM-5 H-mordenite. In general endo is the major product because it is formed when the electron-withdrawing groups of the dienophile are pointing towards the π electrons of the diene. The actual product formed is the endo adduct. The prefix endo is reserved for the isomer with the substituent located closest or syn to the longest bridge.
 Source: j-tradition.com
Source: j-tradition.com
Exothermic and endothermic reactions When a chemical reaction occurs energy is transferred to or from the surroundings. There is usually a temperature change. In the second example suprafacial approach of a dienophile to the 25 carbons of transtrans -24-hexadiene is seen to lead to a product with two methyl groups on the same side of the cyclohexene ring. It is possible to convert the endo isomer 5b to the exo isomer 5a by an epimerization process through the enol.
 Source: j-tradition.com
Source: j-tradition.com
The prefix exo is reserved for the isomer with the substituent located farthest or anti to the longest bridge. The prefix exo is reserved for the isomer with the substituent located farthest or anti to the longest bridge. It is possible to convert the endo isomer 5b to the exo isomer 5a by an epimerization process through the enol. Surface passivation of Hβ confirms that the reaction proceeds in the inner channels.
If it is orientated away from the highest numbered. This is tentatively assigned as the endo isomer and on base treatment it isomerizes to a new compound identified as the exo isomer. This video also helps you to see when you get 1 or 2 different constitutional isomers. In the second example suprafacial approach of a dienophile to the 25 carbons of transtrans -24-hexadiene is seen to lead to a product with two methyl groups on the same side of the cyclohexene ring.
In the first example dimethyl cis -butadioate adds to 13-butadiene to give a cis-substituted cyclohexene.
If the group is attached to the highest numbered. This is because although the hydrogens of the maleic anhydride must be cis in the product there are two possible arrangements where this is true. If it is orientated away from the highest numbered. Translations in context of endo in English-German from Reverso Context. 3 This is because of the interaction and coupling with the H-5 and H-6 as displayed in Figure 1.
 Source: onlinelibrary.wiley.com
Source: onlinelibrary.wiley.com
The exo isomer would possess a triplet around 350 ppm due to the difference in dihedral angle between the hydrogen molecules of H-1 and H-4 and H-5 and H-6 Figure 1. The ENDO product is the one where the outside groups on the diene are on the SAME side of the 6-membered ring as the electron withdrawing group EWG. The prefix endo is reserved for the isomer with the substituent located closest or syn to the longest bridge. It is possible to convert the endo isomer 5b to the exo isomer 5a by an epimerization process through the enol. Here longest and shortest refer to the.
Translations in context of endo in English-German from Reverso Context. For example when a bonfire burns it. And is orientated towards the lowest numbered. Translations in context of endo in French-English from Reverso Context.
This is because although the hydrogens of the maleic anhydride must be cis in the product there are two possible arrangements where this is true.
Here longest and shortest refer to the. The exo isomer would possess a triplet around 350 ppm due to the difference in dihedral angle between the hydrogen molecules of H-1 and H-4 and H-5 and H-6 Figure 1. The prefix exo is reserved for the isomer with the substituent located closest or syn to the shortest bridge. Among the zeolites tested the activity order is Hβ HY HUSY HZSM-5 H-mordenite.
 Source: exo2020reborn.blogspot.com
Source: exo2020reborn.blogspot.com
The prefix exo is reserved for the isomer with the substituent located farthest or anti to the longest bridge. The prefix exo is reserved for the isomer with the substituent located farthest or anti to the longest bridge. If you think about it you can see that when two rings fuse together to make a third four new stereocenters can be created. Giga-fren In sharp contrast to their 321024 analogs the endo isomer is stable while the exo readily rearranges to.
 Source: j-tradition.com
Source: j-tradition.com
What does endo-isomer mean. Beta and Y-type zeolites exhibit higher activity because of their large three-dimensional channels. There is usually a temperature change. The actual product formed is the endo adduct.
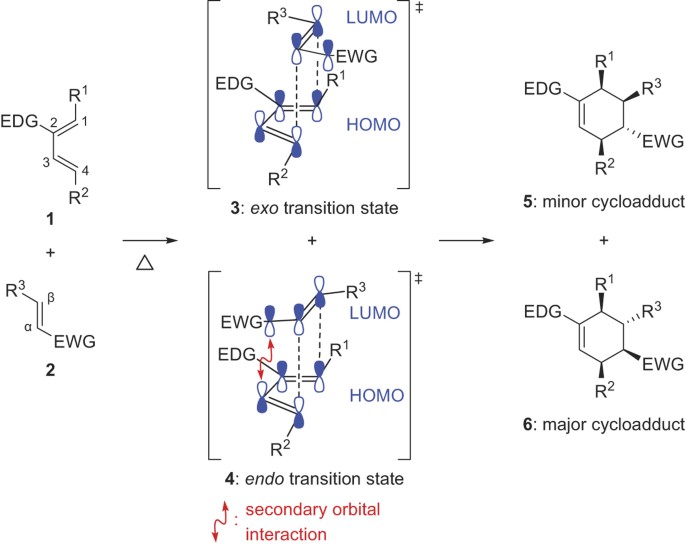 Source: nature.com
Source: nature.com
And is orientated towards the lowest numbered. 3 This is because of the interaction and coupling with the H-5 and H-6 as displayed in Figure 1. It is given the description endo. The Diels-Alder reaction between cyclopentadiene and maleic anhydride can produce two possible products the endo and the exo adducts.
In general endo is the major product because it is formed when the electron-withdrawing groups of the dienophile are pointing towards the π electrons of the diene.
In the first example dimethyl cis -butadioate adds to 13-butadiene to give a cis-substituted cyclohexene. Here longest and shortest refer to the. Endoexo isomerism is a special type of stereoisomerism found in organic compounds with a substituent on a bridged ring system. This is because although the hydrogens of the maleic anhydride must be cis in the product there are two possible arrangements where this is true. This is tentatively assigned as the endo isomer and on base treatment it isomerizes to a new compound identified as the exo isomer.
 Source: exo2020reborn.blogspot.com
Source: exo2020reborn.blogspot.com
In the first example dimethyl cis -butadioate adds to 13-butadiene to give a cis-substituted cyclohexene. Among the zeolites tested the activity order is Hβ HY HUSY HZSM-5 H-mordenite. Organic chemistry An isomer of a bridged organic compound in which a particular substituent is located closest to the. A detergent comprising tetrahydrodicyclopentadiene in which the endoexo isomer ratio by weight is from 7030 to 0100. In the endo product the substituents of the dienophile are pointing towards the larger bridge while in the exo isomer they are pointing away from the larger bridge.
The Diels-Alder reaction between cyclopentadiene and maleic anhydride can produce two possible products the endo and the exo adducts.
Organic chemistry An isomer of a bridged organic compound in which a particular substituent is located closest to the. And is orientated towards the lowest numbered. If you think about it you can see that when two rings fuse together to make a third four new stereocenters can be created. The ENDO product is the one where the outside groups on the diene are on the SAME side of the 6-membered ring as the electron withdrawing group EWG.
 Source: onlinelibrary.wiley.com
Source: onlinelibrary.wiley.com
The prefix exo is reserved for the isomer with the substituent located farthest or anti to the longest bridge. The endo and exo products are really two different diastereomers. If the group is attached to the highest numbered. The EXO product is the one where the.
 Source: nature.com
Source: nature.com
And is orientated towards the lowest numbered. Surface passivation of Hβ confirms that the reaction proceeds in the inner channels. The endo- to exo-isomerization of dicyclopentadiene was performed in liquid phase using acidic zeolites. The actual product formed is the endo adduct.
 Source: j-tradition.com
Source: j-tradition.com
For example when a bonfire burns it. Beta and Y-type zeolites exhibit higher activity because of their large three-dimensional channels. It is possible to convert the endo isomer 5b to the exo isomer 5a by an epimerization process through the enol. Since each chiral centre could have two possible configurations there are sixteen possible stereoisomers that could result in the reaction shown above.
Surface passivation of Hβ confirms that the reaction proceeds in the inner channels.
The EXO product is the one where the. The endo and exo products are really two different diastereomers. The prefix endo is reserved for the isomer with the substituent located closest or syn to the longest bridge. In general endo is the major product because it is formed when the electron-withdrawing groups of the dienophile are pointing towards the π electrons of the diene. The prefix endo is reserved for the isomer with the substituent located closest or syn to the longest bridge.
 Source: europepmc.org
Source: europepmc.org
In the second example suprafacial approach of a dienophile to the 25 carbons of transtrans -24-hexadiene is seen to lead to a product with two methyl groups on the same side of the cyclohexene ring. Among the zeolites tested the activity order is Hβ HY HUSY HZSM-5 H-mordenite. 3 This is because of the interaction and coupling with the H-5 and H-6 as displayed in Figure 1. The prefix endo is reserved for the isomer with the substituent located closest or syn to the longest bridge. Translations in context of endo in English-German from Reverso Context.
What does endo-isomer mean.
Here longest and shortest refer to the. What does endo-isomer mean. The exo isomer would possess a triplet around 350 ppm due to the difference in dihedral angle between the hydrogen molecules of H-1 and H-4 and H-5 and H-6 Figure 1. The prefix endo is reserved for the isomer with the substituent located closest or syn to the longest bridge.
 Source: j-tradition.com
Source: j-tradition.com
The stereochemistry of the products are also discussed such as the cis and trans diastereomer isomers that can be formed in addition to enantiomers and meso compounds. What does endo-isomer mean. Giga-fren In sharp contrast to their 321024 analogs the endo isomer is stable while the exo readily rearranges to. The endo- to exo-isomerization of dicyclopentadiene was performed in liquid phase using acidic zeolites. The EXO product is the one where the.
 Source: j-tradition.com
Source: j-tradition.com
What does endo-isomer mean. The endo and exo products are really two different diastereomers. Since each chiral centre could have two possible configurations there are sixteen possible stereoisomers that could result in the reaction shown above. If it is orientated away from the highest numbered. Translations in context of endo in English-German from Reverso Context.
 Source: exo2020reborn.blogspot.com
Source: exo2020reborn.blogspot.com
The prefix endo is reserved for the isomer with the substituent located closest or syn to the longest bridge. C-7 in example below it is given the description exo. Translations in context of endo in English-German from Reverso Context. Two examples follow which are drawn to emphasize how suprafacial addition occurs. This is because although the hydrogens of the maleic anhydride must be cis in the product there are two possible arrangements where this is true.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site beneficial, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title endo and exo isomers examples by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





