Diels alder exo endo.
If you’re searching for diels alder exo endo pictures information related to the diels alder exo endo interest, you have pay a visit to the right site. Our website frequently gives you hints for seeing the maximum quality video and picture content, please kindly surf and locate more informative video content and images that match your interests.
 Organic Chemistry Ii Dr Christopher Cioffi Monday 3202017 From slidetodoc.com
Organic Chemistry Ii Dr Christopher Cioffi Monday 3202017 From slidetodoc.com
The Diels-Alder addition of cyclopropenone and 1-3-diphenylisobenzofuran yields only the exo adduct Figure 11. The intramolecular ionic DielsAlder IIDA reactions of two dieniminiums were studied within the Molecular Electron Density Theory MEDT at the ωB97XD6-311Gdp computational level. This looks ordinary until we draw the product from a side view which reveals some nice structures and interesting features of the mechanism that leads to the formation of two stereoisomers. Synchronous and symmetrical concerted mechanisms when.
The authors Cordes de Gala and Berson compare this exo-endo transformation to another rearrangement on the cyclopropanone shown on.
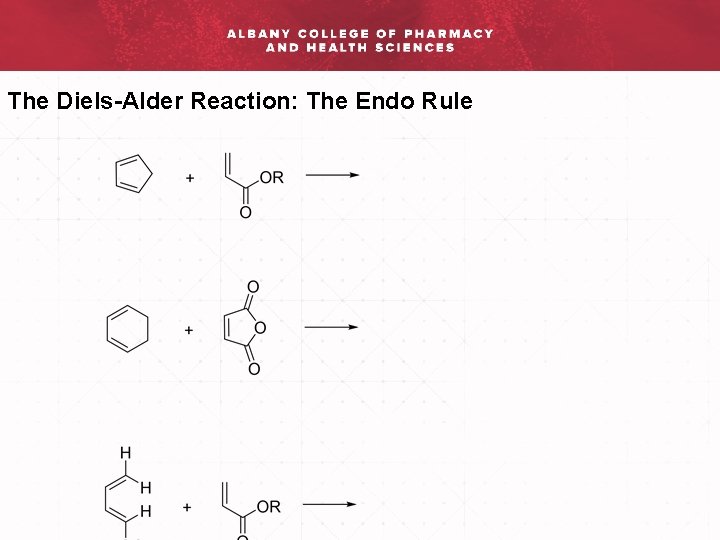
When a cyclic diene is used in the Diels-Alder reaction a bridged bicyclic compound is formed. There is not a single mechanism for all Diels-Alder reactions. Pay Attention To The Relationship Between The Outside Groups On The Diene And The EWG On The Dienophile. Often there are already rings in the molecules undergoing reaction and a new one is being added. The Diels-Alder reaction is a thermal cycloaddition whose mechanism involves the sigma-overlap of the pi-orbitals of the two unsaturated systems.
 Source: slideplayer.com
Source: slideplayer.com
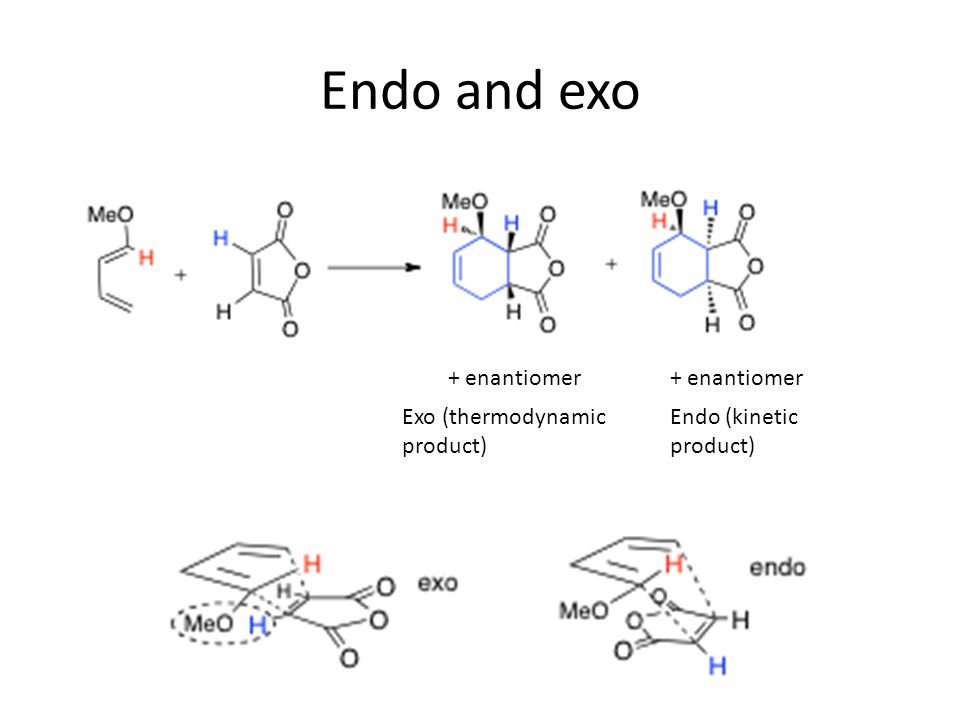
The intramolecular ionic DielsAlder IIDA reactions of two dieniminiums were studied within the Molecular Electron Density Theory MEDT at the ωB97XD6-311Gdp computational level. Exo And Endo Diels-Alder Products Are Diastereomers Of Each Other 3-D Models Of The Endo And Exo Products In The Diels-Alder Between Cyclopentadiene And Maleic Anhydride Distinguishing Endo vs Exo. Endo and Exo Products. This looks ordinary until we draw the product from a side view which reveals some nice structures and interesting features of the mechanism that leads to the formation of two stereoisomers. It allows the construction of six-membered rings which are very common in biological small molecules which are frequently synthetic targets.
The Diels-Alder addition of cyclopropenone and 1-3-diphenylisobenzofuran yields only the exo adduct Figure 11.
The rate at which a Diels-Alder reaction takes place depends on electronic as. The endo position on a bicyclic structure refers to the position that is inside the concave shape of the larger six-membered ring. In this case the exo product is thermodynamically favored over the endo product by about 19 k c a l m o l. The intramolecular ionic DielsAlder IIDA reactions of two dieniminiums were studied within the Molecular Electron Density Theory MEDT at the ωB97XD6-311Gdp computational level.
 Source: pinterest.com
Source: pinterest.com
Synchronous and symmetrical concerted mechanisms when. It turns out that the rate of formation of the expected endo product is actually 500 times faster than the rate of formation of the exo product. The Diels Alder reaction is probably the most common cycloaddition. There is not a single mechanism for all Diels-Alder reactions.
 Source: youtube.com
Source: youtube.com
Diels-Alder orbital explanation for the endo rule. Pay Attention To The Relationship Between The Outside Groups On The Diene And The EWG On The Dienophile. It turns out that the rate of formation of the expected endo product is actually 500 times faster than the rate of formation of the exo product. This is why it forms more quickly.
 Source: slideplayer.com
Source: slideplayer.com
Use getProperty modelInfo or getProperty auxiliaryInfo to inspect them. Use getProperty modelInfo or getProperty auxiliaryInfo to inspect them. In most cases the endo product is consid. Reactions Under Orbital Control.
In most cases the endo product is consid. Often there are already rings in the molecules undergoing reaction and a. Exo And Endo Diels-Alder Products Are Diastereomers Of Each Other 3-D Models Of The Endo And Exo Products In The Diels-Alder Between Cyclopentadiene And Maleic Anhydride Distinguishing Endo vs Exo. There is not a single mechanism for all Diels-Alder reactions.
Endo and Exo Products.
Often there are already rings in the molecules undergoing reaction and a new one is being added. I have explained the complete mechanism with the help. This reaction was first made by Breslow and Oda. Diels-Alder orbital explanation for the endo rule. In most cases the endo product is consid.
 Source: pinterest.com
Source: pinterest.com
The rate at which a Diels-Alder reaction takes place depends on electronic as. In most cases the endo product is consid. Topological analysis of the electron localization function ELF of dieniminiums showed that their electronic structures can been seen as the sum of those of butadiene and ethaniminium. This is why it forms more quickly. This looks ordinary until we draw the product from a side view which reveals some nice structures and interesting features of the mechanism that leads to the formation of two stereoisomers.
The rate at which a Diels-Alder reaction takes place depends on electronic as. When a cyclic diene is used in the Diels-Alder reaction a bridged bicyclic compound is formed. The cyclo-reversion temperature of the first one is lower than the exo adduct and the ratio between endo and exo adducts varies according to the substituents of the DielsAlder. The intramolecular ionic DielsAlder IIDA reactions of two dieniminiums were studied within the Molecular Electron Density Theory MEDT at the ωB97XD6-311Gdp computational level.
When a cyclic diene is used in the Diels-Alder reaction a bridged bicyclic compound is formed.
In a recent publication it was proposed that this exo preference is due to a thremodynamic effect. The endo position on a bicyclic structure refers to the position that is inside the concave shape of the larger six-membered ring. Often there are already rings in the molecules undergoing reaction and a new one is being added. The cyclo-reversion temperature of the first one is lower than the exo adduct and the ratio between endo and exo adducts varies according to the substituents of the DielsAlder.
 Source: youtube.com
Source: youtube.com
The intramolecular ionic DielsAlder IIDA reactions of two dieniminiums were studied within the Molecular Electron Density Theory MEDT at the ωB97XD6-311Gdp computational level. I have explained the complete mechanism with the help. The authors Cordes de Gala and Berson compare this exo-endo transformation to another rearrangement on the cyclopropanone shown on. In a recent publication it was proposed that this exo preference is due to a thremodynamic effect.
 Source: pinterest.com
Source: pinterest.com
In this lecture I have discussed the Diels-Alder reaction and selectivity for endo or exo product. In the Diels-Alder reaction the Endo and Exo products are formed when a cyclic diene is reacted with a dienophile. In this case the exo product is thermodynamically favored over the endo product by about 19 k c a l m o l. The rate at which a Diels-Alder reaction takes place depends on electronic as.
 Source: exo2020reborn.blogspot.com
Source: exo2020reborn.blogspot.com
Endo and Exo Products. The Diels-Alder reaction is a thermal cycloaddition whose mechanism involves the sigma-overlap of the pi-orbitals of the two unsaturated systems. In most cases the endo product is consid. It turns out that the rate of formation of the expected endo product is actually 500 times faster than the rate of formation of the exo product.
Pay Attention To The Relationship Between The Outside Groups On The Diene And The EWG On The Dienophile.
I have explained the complete mechanism with the help. Synchronous and symmetrical concerted mechanisms when. However the DielsAlder is a reversible reaction. The authors Cordes de Gala and Berson compare this exo-endo transformation to another rearrangement on the cyclopropanone shown on. Use getProperty modelInfo or getProperty auxiliaryInfo to inspect them.
 Source: youtube.com
Source: youtube.com
Use getProperty modelInfo or getProperty auxiliaryInfo to inspect them. Reactions Under Orbital Control. Topological analysis of the electron localization function ELF of dieniminiums showed that their electronic structures can been seen as the sum of those of butadiene and ethaniminium. Often there are already rings in the molecules undergoing reaction and a. The endo product must have a lower energy transition state not final structure than the exo product.
Often there are already rings in the molecules undergoing reaction and a new one is being added.
Topological analysis of the electron localization function ELF of dieniminiums showed that their electronic structures can been seen as the sum of those of butadiene and ethaniminium. The cyclo-reversion temperature of the first one is lower than the exo adduct and the ratio between endo and exo adducts varies according to the substituents of the DielsAlder. The authors Cordes de Gala and Berson compare this exo-endo transformation to another rearrangement on the cyclopropanone shown on. I have explained the complete mechanism with the help.
 Source: slideplayer.com
Source: slideplayer.com
In most cases the endo product is consid. The cyclo-reversion temperature of the first one is lower than the exo adduct and the ratio between endo and exo adducts varies according to the substituents of the DielsAlder. A The expected endo 9 and exo 10 adducts formed from the substrate analogue 8 by the spontaneous or enzyme-catalysed DielsAlder reaction. Often there are already rings in the molecules undergoing reaction and a.
 Source: youtube.com
Source: youtube.com
Reactions Under Orbital Control. It turns out that the rate of formation of the expected endo product is actually 500 times faster than the rate of formation of the exo product. The Diels-Alder addition of cyclopropenone and 1-3-diphenylisobenzofuran yields only the exo adduct Figure 11. I have explained the complete mechanism with the help.
 Source: exo2020reborn.blogspot.com
Source: exo2020reborn.blogspot.com
The Diels Alder reaction is probably the most common cycloaddition. This reaction was first made by Breslow and Oda. In the Diels-Alder reaction the Endo and Exo products are formed when a cyclic diene is reacted with a dienophile. As you might predict the exo position refers to the outside position.
In this lecture I have discussed the Diels-Alder reaction and selectivity for endo or exo product.
Endo and Exo Products. Topological analysis of the electron localization function ELF of dieniminiums showed that their electronic structures can been seen as the sum of those of butadiene and ethaniminium. When a cyclic diene is used in the Diels-Alder reaction a bridged bicyclic compound is formed. The rate at which a Diels-Alder reaction takes place depends on electronic as. This reaction was first made by Breslow and Oda.
 Source: pinterest.com
Source: pinterest.com
20 models in this collection. Topological analysis of the electron localization function ELF of dieniminiums showed that their electronic structures can been seen as the sum of those of butadiene and ethaniminium. In this case the exo product is thermodynamically favored over the endo product by about 19 k c a l m o l. The intramolecular ionic DielsAlder IIDA reactions of two dieniminiums were studied within the Molecular Electron Density Theory MEDT at the ωB97XD6-311Gdp computational level. Exo And Endo Diels-Alder Products Are Diastereomers Of Each Other 3-D Models Of The Endo And Exo Products In The Diels-Alder Between Cyclopentadiene And Maleic Anhydride Distinguishing Endo vs Exo.
It turns out that the rate of formation of the expected endo product is actually 500 times faster than the rate of formation of the exo product.
When a cyclic diene is used in the Diels-Alder reaction a bridged bicyclic compound is formed. As you might predict the exo position refers to the outside position. The cyclo-reversion temperature of the first one is lower than the exo adduct and the ratio between endo and exo adducts varies according to the substituents of the DielsAlder. The endo product must have a lower energy transition state not final structure than the exo product.
 Source: pinterest.com
Source: pinterest.com
Endo and Exo products of Diels-Alder Reaction with Practice Problems - Chemistry Steps. Use getProperty modelInfo or getProperty auxiliaryInfo to inspect them. Often there are already rings in the molecules undergoing reaction and a new one is being added. This reaction was first made by Breslow and Oda. It allows the construction of six-membered rings which are very common in biological small molecules which are frequently synthetic targets.
 Source: slideplayer.com
Source: slideplayer.com
This looks ordinary until we draw the product from a side view which reveals some nice structures and interesting features of the mechanism that leads to the formation of two stereoisomers. 20 models in this collection. The key difference between Endo and Exo Diels Alder compounds is that the Endo Diels Alder product has its substituents on the same face of the bridged ring system whereas the Exo Diels Alder product has its substituents on the opposite faces of the bridged ring system. In this case the exo product is thermodynamically favored over the endo product by about 19 k c a l m o l. Reactions Under Orbital Control.
 Source: youtube.com
Source: youtube.com
Endo and Exo Products. Endo and Exo products of Diels-Alder Reaction with Practice Problems - Chemistry Steps. It allows the construction of six-membered rings which are very common in biological small molecules which are frequently synthetic targets. The rate at which a Diels-Alder reaction takes place depends on electronic as. However the DielsAlder is a reversible reaction.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title diels alder exo endo by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





